Nanocarbon
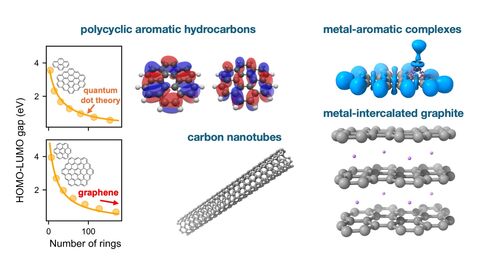
Carbon nanomaterials are relevant across a range of applications. Flame-formed carbon nanoparticles exhibit tunable optical and electronic properties that can be controlled by varying their size and composition. They consist of polycyclic aromatic hydrocarbons, whose properties can be described by 2D Quantum Dot theory. Polycyclic aromatic hydrocarbons, graphene and graphite bind strongly with metals, with the resulting metal-aromatic complexes exhibiting drastically different properties than those of their constituents. Metal-aromatic complexes appear in interstellar dust, accelerate soot nucleation, control fuel coking, and can be utilised in nanoelectronics. Metal-intercalated graphite is the primary battery anode material in use today. Understanding the interaction of metal ions with graphite is crucial in developing future battery anode materials and predicting battery electrochemical operation.
Understanding, modelling and controlling the properties of nanocarbon can help develop next-generation battery materials, detect and reduce combustion emissions, understand interstellar light absorption, model arctic ice darkening, and develop organic nanoelectronics, among other applications.
