Electrochemistry
Designing and optimising high-performance battery systems requires understanding the chemical reactions that govern electrochemical operation. Understanding such reaction mechanism is best achieved via a combined experimental and computational chemistry approach. In addition, battery safety is becoming increasingly important, as large-scale energy storage facilities are necessary for renewable energy storage. When battery cells are operated at elevated temperatures and/or current densities, chemical reactions between the anode, cathode and electrolyte initiate thermal runaway and have the potential to lead to battery fires and explosions.
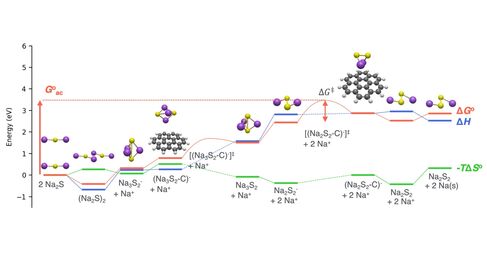
Electrochemical and thermal runaway reactions can exhibit a range of thermodynamic and kinetic behaviours, because they occur over a range of surface sites on liquid-solid interfaces. At each surface site, the chemical potential, electrical potential, work function and solvent structure are different. Understanding global electrochemical and thermal runaway behaviour requires understanding surface-site-resolved thermodynamics and kinetics.
